Physiologically-based kinetics modelling for nanomaterials
Biokinetics offer a methodology for predicting the internal distribution and exposure of a substance in an organism.
Compartmental modelling is a concept broadly used in pharmacokinetics for describing the biodistribution of a substance inside an organism. Physiologically-based pharmacokinetic (PBPK) models represent one of the two major approaches used in compartmental modelling, with empirical models being the second one. PBPK models are mechanistic; they consist of compartments representing real organs and tissues, whose number varies based on the target substance, species, administration route and available information. A common approach is to incorporate in the model the main body tissues, i.e. brain, heart, kidney, skin, spleen, liver, lung, gut, bone, adipose and muscle. In most cases, PBPK models are utilised for describing the kinetics of a substance in the whole body of a species, thus such models are more formally called “whole body physiologically-based pharmacokinetic models” (WBPBPK).
How to simulate biodistribution scenarios using custom PBPK models This document provides a tutorial for simulating biodistribution scenarios using custom PBPK models that have been deployed on Jaqpot5.
WBPBPK can be grouped into two classes: perfusion-limited and permeability-limited models. The first group applies for small lipophilic molecules, where the limiting step of substance absorption is the transportation to the tissue via the blood flow. On the other hand, permeability-limited models assume that the limiting process is the membrane permeability which acts as a diffusion barrier, so it applies for larger and hydrophilic molecules, which is the case for NMs. The compartments of a WBPBPK model are interconnected through the arterial and venous blood pools; all organs receive blood from the arterial blood compartment and the outflow ends up in the venous blood compartment. The blood loop is closed by the lungs compartment, in which the blood flow is reversed in comparison to the rest of the organs. Apart from the regional blood flows, the underlying physiology also defines the second set of substance-independent parameters, the organ volumes. Besides these physiological parameters, PBPK models incorporate information about the target substance as well, through the substance-dependent parameters, which can be obtained using data generated by in vivo, in vitro or in silico experiments.
PBPK models have inherent advantages due to their mechanistic nature. Firstly, they enable predictions of concentration/mass profiles of individual organs and not just of plasma. In addition, their relation with physiology and modularity facilitate the integration of literature information, making predictions prior to in vivo experiments possible. Lastly, their biggest advantage is the ability to perform inter-species (e.g. from rat to human) or intra-species (e.g. from adults to children) extrapolation through scaling methods.
See for example: PBPK modelling on the Jaqpot web platform - a PAA-peg nanoparticles case study
Nano-PBPK models
Several PBPK models that describe the biodistribution of NMs can be found in literature and an extensive review of the modifications of traditional PBPK models that need to be considered in the case of NMs can be found in (Li et al., 2010, Yuan et al., 2019).
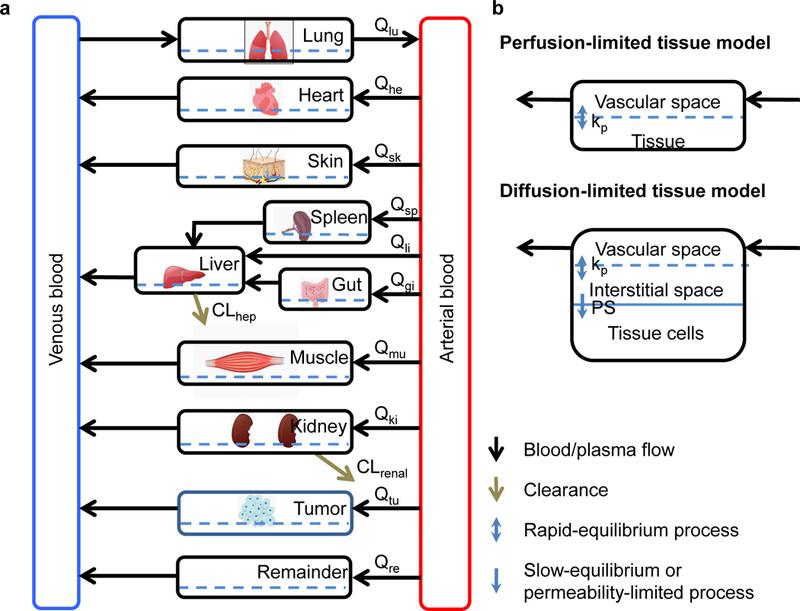
Figure 1. generic PBPK model (A) and two types of tissue model structure (B). Qi: blood or plasma flow; kp: tissue partitioning coefficient, namely concentration ratio between tissue and blood at steady-state; PS: membrane permeability coefficient; CLhep: hepatic clearance; CLrenal: renal clearance (extracted from DOI: 10.1016/j.xphs.2018.10.037
A list containing nano-PBPK models is presented in the table below:
| Nanoparticle | Species | Administration route | Reference |
| Quantum Dot 705 (QD705) | Mouse | Intravenous | https://doi.org/10.1021/es800254a |
| 99mTechnetium - labelled carbon nanoparticles (Technegas) | Male human | Pulmonary | https://doi.org/10.3109/08958370902748542 |
| Silver nanoparticles | Rat | Intravenous | https://doi.org/10.1016/j.biomaterials.2010.07.045 |
| poly(lactic - co - glycolic) acid (PLGA) nanoparticles | Mouse | Intravenous | https://doi.org/10.2147/IJN.S23758 |
| gold/dendrimer composite nanodevices (CNDs) | Mouse | Intravenous | https://doi.org/10.1007/s11095-012-0784-7 |
| Silver, silver alloy and ionic silver | Rat | Intravenous | https://doi.org/10.2147/IJN.S46624 |
| 192Ir radio-labeled iridium particles and ultrafine carbon particles | Rat | Pulmonary | https://doi.org/10.1115/1.4025333 |
| Polyacrylamide (PAA) | Rat | Intravenous | https://doi.org/10.3109/17435390.2013.863406 |
| Gold nanoparticles | Mouse | Intravenous | https://doi.org/10.3109/17435390.2015.1027314 |
| Silver nanoparticles | Rat | Intragastric | https://doi.org/10.1134/S1995078015020081 |
| Titanium dioxide (TiO2) nanoparticles | Mouse, Rat | Intravenous | https://doi.org/10.3109/17435390.2014.940404 |
| Zinc oxide (ZnO) and Zinc Nitrate (Zn(NO3)2) nanoparticles | Mouse | Intravenous | https://doi.org/10.2147/IJN.S86785 |
| Cadmium telluride/cadmium sulphide quantum dots (CdTe/CDS Qds) | Mouse | Intravenous | https://doi.org/10.1021/acs.nanolett.5b03854 |
| Silver and Carbon black (CB) nanoparticles | Mouse | Pulmonary | https://doi.org/10.1371/journal.pone.0080917 |
| PAA, Gold, TiO2 | Rat | Intravenous | https://doi.org/10.2147/IJN.S94370 |
| QD705 | Mouse | Intravenous | https://doi.org/10.1021/nl803481q |
| TiO2 | Rat | Intravenous | https://doi.org/10.1371/journal.pone.0124490 |
| Amphiphilic block copolymers poly (ethylene glycol) (PEG) - caprolactone) (PCL) bearing pendant cyclic ketals | Mouse | Intravenous | https://doi.org/10.1002/psp4.13 |
| Nanocrystals of SNX-2112 | Rat | Intravenous | https://doi.org/10.2147/IJN.S79734 |
| 192Ir radio - labelled iridium and silver nanoparticles | Rat | Pulmonary, Intravenous | https://doi.org/10.1016/j.yrtph.2015.06.019 |
| Gold | Mouse, rat, pig | Intravenous | https://doi.org/10.2217/nnm.15.177 |
| PAA | Rat | Intravenous | https://doi.org/10.1016/j.taap.2010.11.017 |
| 192Ir radio-labelled iridium and silver nanoparticles | Rat | Pulmonary | https://doi.org/10.1088/1742-6596/151/1/012028 |
| Gold | Rat | Pulmonary | https://doi.org/10.1088/1742-6596/151/1/012029 |
| Molecular imaging nanoparticles (MINs) | Mouse | Injection | https://doi.org/10.1089/oli.2009.0216 |
| Cerium dioxide (CeO2) | Rat | Pulmonary | https://doi.org/10.1186/s12989-016-0156-2 |
| CeO2 | Rat | Pulmonary, Intravenous, oral or its instillation | https://doi.org/10.2147/IJN.S157210 |
| Superparamagnetic iron oxide nanoparticles (SPIONs) | Mouse | Intravenous | https://doi.org/10.1515/ejnm-2017-0001 |
Nano-PBPK web services
NTUA has developed all the necessary infrastructure to develop, host and share PBPK models through the Jaqpot computational platform, thus, a series of models have been integrated with the NanoCommons KnowledgeBase through the Jaqpot Cloud Platform and can be readily integrated in risk assessment computational pipelines. Potential users can learn how to acquire predictions from these models by visiting the detailed documentation files that have been uploaded in zenodo:
Jaqpot 5: How to simulate biodistribution scenarios using custom PBPK models This document provides a tutorial for simulating biodistribution scenarios using custom PBPK models that have been deployed on Jaqpot5.
To acquire access to the models, a user should be part of the NanoCommons organisation in Jaqpot. For this purpose, email Prof. Harry Sarimveis requesting access (hsarimv@central.ntua.gr)
PBPK model on polyethylene glycol-coated polyacrylamide (PAA-peg) nanoparticles on rat
This PBPK model has been developed on a rat population to describe the biodistribution of polyethylene glycol-coated (PAA-peg) nanoparticles. It consists of 7 compartments describing the mass distribution of the nanoparticles in various organs, namely liver (LI), kidney (KI), brain (BR), bone marrow (BM), , heart (HT), spleen (SPL) and lungs (LU), one compartment to model the rest of the body (ROB) as well as two blood pools; venous (VEN) and arterial (ART). All compartments include a sub-compartment describing the uptake of nanoparticles by phagocytizing cells (PCs), while all but the blood compartments have a third component describing the distribution of nanoparticles in the capillary blood.The detailed description of the PBPK model used can be found in Li et al., 2014.
The model can be accessed through: https://app.jaqpot.org/model/lUP5Nqoa6JOTZG4CQnSJ
PBPK model on Titanium dioxide (TiO2) nanoparticles on mice
This PBPK model was initially developed on rat data Kreyling et al., 2019 to describe the biodistribution of TiO2 nanoparticles after inhalation. After adaptation to the biodistribution data, the model was extrapolated to mice using scaling of physiological parameters and MPPD for extrapolating parameters related to inhalation. Its schematic representation can be seen in the following figure. It consists of 10 compartments describing the mass distribution of the nanoparticles in various organs, namely liver, kidney, brain, gonads, heart, spleen, lungs, skin, skeleton, one compartment to model the soft tissues, which acts as a rest of the body compartment, as well as two blood pools; venous and arterial. Because the model refers to inhalation, a more detailed description of the respiratory tract was implemented, with analytical equations for the upper airways, tracheobronchial and alveolar regions. Most compartments include a sub-compartment describing the uptake of nanoparticles by phagocytizing cells (PCs), while all compartments connected to the systemic circulation have a third component describing the distribution of nanoparticles in the capillary blood.The structural model was built upon the PBPK of Li et al., 2016. This model was developed using financial support by NanoCommons (Grant Agreement 731032), a project funded by the European Commission under the Horizon 2020 Programme.
The Model can be accessed through: https://app.jaqpot.org/model/3xz9DIdtuIRw2qaj3kGf
Naive Human PAA-PEG PBPK Model
This PBPK model is a human extrapolation of the Li et al., 2014 PBPK model and describes the biodistribution of polyethylene glycol-coated polyacrylamide (PAA-peg) nanoparticles. The set of physiological parameters which were changed from the original values of Li et al., 2014 are the weights, the volume of capillary blood and the blood flow of each tissue/organ. The values of these parameters are provided by Brown et al., 1997.
The PBPK model consists of 7 compartments describing the mass distribution of the nanoparticles in various organs, namely liver (LI), kidney (KI), brain (BR), bone marrow (BM), heart (HT), spleen (SPL) and lungs (LU), one compartment to model the rest of the body (ROB) as well as two blood pools; venous (VEN) and arterial (ART). All compartments include a sub-compartment describing the uptake of nanoparticles by phagocytizing cells (PCs), while all but the blood compartments have a third component describing the distribution of nanoparticles in the capillary blood.
The model can be accessed through: https://app.jaqpot.org/model/n1O9U65PxciUPIJBad0S
PBK model for 99m-Technetium-labelled carbon nanoparticles inhaled by humans
This Physiologically Based Kinetic (PBK) model has been developed using radioactivity data resulting from inhalation of technetium- labelled carbon nanoparticles (5-10 nm) from healthy male volunteers (24 to 47 years old). The detailed description of the PBK model can be found in Pery et al., 2009.
The model considers a concentration for technetium bound to small particles, in other words labelled nanoparticles, and free technetium. Hence the web service reports both the concentration of free and bound technetium in each compartment, as well as the total technetium concentration which is calculated as the summation of the two technetium forms. The concentration that is related to technetium bound to large particles cannot translocate the air:blood barrier and, thus, only the bound and total amounts of the lungs and stomach lumen ( through translocation from the lungs) contain radiation intensity from technetium bound to large particles, which is considered constant over time.
The model can be accessed through: https://app.jaqpot.org/model/KSO7Or1TJVlVuntsSIUK
References
- Brown et al., 1997: Brown, R.P.; Delp, M.D.; Lindstedt, S.L.; Rhomberg, L.R.; Beliles, R.P. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 1997, 13(4), 407–84. https://doi.org/10.1177/074823379701300401
- Carlander et al., 2016: Carlander, U.; Li, D.; Jolliet, O.; Emond, C.; Johanson, G.; Toward a general physiologically-based pharmacokinetic model for intravenously injected nanoparticles. Int J Nanomed 2016, 11, 625–640. https://doi.org/10.2147/IJN.S94370
- Kreyling et al., 2019: Kreyling, W.G.; Holzwarth, U.; Schleh, C. et al. Quantitative biokinetics over a 28 day period of freshly generated, pristine, 20 nm titanium dioxide nanoparticle aerosols in healthy adult rats after a single two-hour inhalation exposure. Part Fibre Toxicol. 2019, 16, 29. https://www.doi.org/10.1186/s12989-019-0303-7
- Li et al., 2014: Li, D.; Johanson, G.; Emond, C.; Carlander, U.; Philbert, M.; Jolliet, O.; Physiologically based pharmacokinetic modeling of polyethylene glycol-coated polyacrylamide nanoparticles in rats. Nanotoxicology 2014, 8, 128–137. https://doi.org/10.3109/17435390.2013.863406
- Li et al., 2016: Li, D.; Morishita, M.; Wagner, J.G.; et al. In vivo biodistribution and physiologically based pharmacokinetic modeling of inhaled fresh and aged cerium oxide nanoparticles in rats. Part Fibre Toxicol. 2016, 13(1), 45. https://www.doi.org/10.1186/s12989-016-0156-2
- Li et al., 2010: Li, M.; Al-Jamal, K.T.; Kostarelos, K.; Reineke, J. Physiologically Based Pharmacokinetic Modeling of Nanoparticles. ACS Nano 2010, 4(11), 6303–6317. https://doi.org/10.1021/nn1018818
- Pery et al., 2009: Pery, A.R.R.; Brochot, C.; Hoet, P.H.M.; Nemmar, A.; Bois, F.Y. Development of a physiologically based kinetic model for 99m-Technetium-labelled carbon nanoparticles inhaled by humans. Inhalation Toxicology 2009, 21(13), 1099-1107. https://doi.org/10.3109/08958370902748542
- Yuan et al., 2019: Yuan, D.; He, H.; Wu, Y.; Fan, J.; Cao, Y. Physiologically Based Pharmacokinetic Modeling of Nanoparticles. J Pharm Sci 2019, 108(1), 58–72. https://doi.org/10.1016/j.xphs.2018.10.037