NanoRisk Governance
Setting up as a bridge between regulatory science and policy
Table of contents
Introductory training on risk governance From the 10th Nanosafety Training School: from Basic Science to Risk Governance in Venice 2021, 21-25 June 2021
Nano-Risk Governance Portal Much more information on risk governance is available from the three NMBP-13 projects RiskGONE, NANORIGO and Gov4Nano. It covers guidance, a list of tools, available data, and related platforms (including this Handbook sentiment_satisfied).
Significant progress has been achieved in relation to research regarding the safety of engineered nanomaterials and the transfer of this knowledge into regulation. Still, more needs to be done as nanotechnology reaches the market. To fill this gap, transdisciplinary risk governance is required based on a clear understanding of risk, its management practices and the societal risk perception by all stakeholders. It should propose and apply clear criteria for risk evaluation and acceptance and for transfer of acceptable risk. It should develop reinforced decision making tools incorporating those aspects and facilitate risk communication to relevant stakeholders, including industry, regulators, insurance companies and the general public.
Scope:
- Data and information management and framework tools with regard to the safety of nanomaterials for risk assessment, hazard and exposure, human health and environment, and risk mitigation including regulatory aspects of safe-by-design;
- Responsible communication with stakeholders and the civil society based on good quality information and valuable feedback;
- Plans for future scientific and regulatory research paying attention to social, ethical and environmental aspects, to achieve completeness, consistency, maximum synergy of actions and international cooperation;
- Mechanisms to monitor progress in several industrial sectors and to revise plans.
(reproduced from the H2020 call text Risk Governance of nanotechnology (RIA), TOPIC ID: NMBP-13-2018)
A crucial element related to risk governance is the establishment, by consensus, of a set of scientifically reliable and regulatory relevant technical guidelines and good practices documents. Ideally such guidelines and documents should be aligned to the requirements for guidance and standards of international organisations such as OECD, ISO and CEN. Agreed standardised test guidelines and guidance documents are needed to allow reliable and relevant safety testing of nanomaterials regarding both human health and the environment. In particular, the OECD guidelines for chemicals’ notification and registration under REACH need adaptation for nanomaterials from characterisation of materials and exposure, to potential for persistence, bioaccumulation and toxicity.
Scope:
- Outline specific research actions of regulatory research nature to cover existing gaps in OECD test guidelines and guidance documents development;
- Establish integration of other public and private resources (funding or labour) to develop and validate new OECD test guidelines and OECD guidance documents;
- Establish maximum synergy of actions across industrial sectors and international cooperation;
- Support the completion of the elaborated documents by the relevant international organisations involving OECD Member States and relevant EU agencies;
- Establish very close cooperation with Member States, OECD, BIAC, JRC, ECHA, EU and Member State agencies to act as leads and co-leads for the test guidelines and guidance documents to be developed.
(reproduced from the H2020 call text In support of documentary standards (CSA), TOPIC ID: NMBP-34-2019)
Why is NanoRisk Governance needed
Topics covered:
- What do we need a NRG Council for, who are its stakeholders and what are their needs?
- What can we expect from a future NRG Council and what is the current view on its design?
- Screening ethical issues for governance of nanorisk
- Insight into the nanorisk governance process guided by the NanoRigo NRG framework
- Requirements on NRG frameworks by insurances and other societal stakeholders
OECD Test Guidelines and Guidance Documents
OECD Test Guidelines for Chemicals Access to all OECD Test Guidelines highlighting newly released guidelines, updates and corrections.
Draft documents for public comments
OECD Webinar Series on Testing and Assessment Methodologies The OECD webinars on Testing and Assessment Methodologies focus on selected ongoing projects that will result in the short to medium term in new methodologies to test and assess chemicals. These free webinars are open to potential end users among regulators and contract research organisations who want to get familiar with new standards and approaches.
NanoHarmony: Towards harmonized test methods for nanomaterials
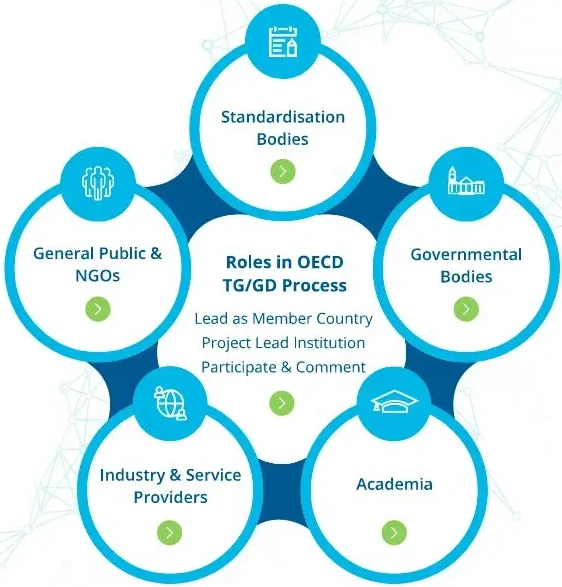
The NanoHarmony project supported the development of OECD Test Guidelines and Guidance Documents for eight endpoints where nanomaterial-adapted test methods have been identified as a regulatory priority. Based on this work, it provides recommendations to address issues, which might jeopardise keeping pace with new scientific developments, to ensure engagement of all the relevant stakeholders, not least those in the academic community, and to make the process of TG development more effective.
White Paper: From Science to Regulation
OECD TG/GD Process Mentor The NanoHarmony Process Mentor provides guidance and understanding on developing OECD Test Guidelines (TGs) and Guidance Documents (GDs), including when and how to prepare for required activities, highlighting key start and finish dates of the development process, plus who to involve in which activities and when.
NanoHarmony training and event recordings
NMBP 13 final online event: Future-proof Approaches for Risk Governance
Lessons Learned from Nanotechnology
Tools for NanoRisk Governance
Tools listed on the Nano-Risk Governance Portal
Data listed on the Nano-Risk Governance Portal
Joint glossary of key terms related to data management and knowledge infrastructure / portal / platform The glossary resulted from activities of the Core Group on Data Management organized jointly by the three projects funded under the H2020 NMBP-13 call: Gov4Nano, NANORIGO and RiskGONE.