Data/knowledge management: general and nanosafety-specific aspects
The data management life cycle
Based on: Papadiamantis et al., 2020
© 2020 by the authors. Licensee MDPI
Despite its significance, structured (meta)data capturing and managing to allow retrieval and sharing (by yourself, your team or the public) is not widely implemented in everyday’s scientific practice. This is due to the general perception of data management as being something done for others postponed to the end of a study / project and after the data are fully analysed and published. As a result, data curators accessing original data files or extracting data from a publication are often unable to locate the information needed to fully implement the necessary metadata. Therefore, the first critical change needed is to shift the design and implementation of data management workflows from project end to project outset. It is imperative to encourage (meta)data upload to data repositories after its creation as depicted in the figure below. This can be implemented with appropriate access restrictions in place to protect intellectual properties where necessary, and with automatic release data following publication. As a result of the cyclical nature of the data lifecycle, it is always possible to optimise the (meta)data coverage, taking into account the needs of different users and re-users.
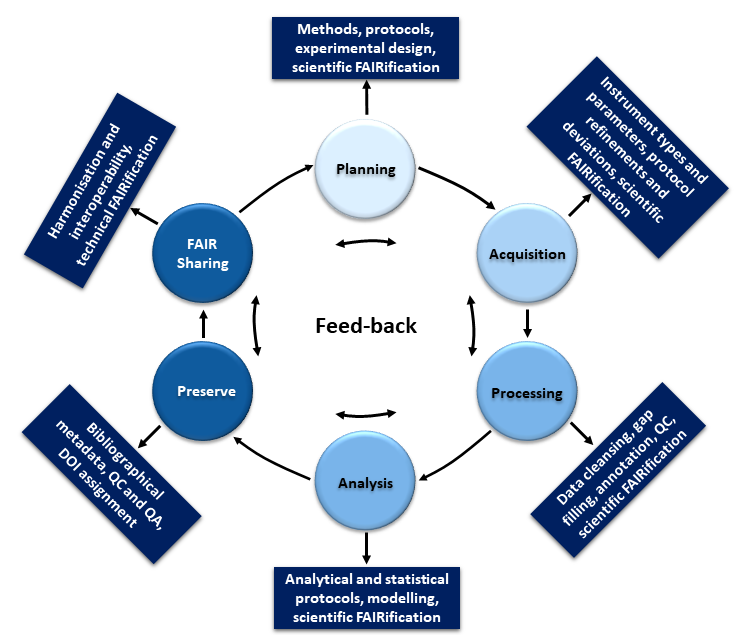
The resources below will give guidance how such a distributed (meta)data collection and management can be organised for the nanosafety and nanoinformatic areas:
Data completeness, minimum information checklist, data quality
Nanosafety data/knowledge resources
Did we miss something (we definitely did)? Please let us know.
Click here to edit the mural below.
And some big resources on data management more generally and neighbouring areas:
Open Science Training Handbook
A Guide to Sharing the Data and Benefits of Public Health Surveillance
Additionally, the Demonstration Cases highlight specific aspects like study design and SOPs and implementation:
ELNs for data collection and annotation This case demonstrates how electronic lab notebooks (ELNs) can be used to manage complex studies and collaborative research. It will also show current challenges especially with respect to the initial high effort to set up the customised workflows, integration with other data management tools and additional requirements when using in regulatory setting. (ended)
Protein corona modelling Bio-nano interaction data (characterization of nanomaterials with and without allergens, structural alterations upon nanoparticle binding, selective binding of allergens from protein mixes, investigations of protein orientations when bound to NMs, etc.) are generated in different past and ongoing projects. This demonstration case was designed around this research to see how corona modelling can help to understand the effects. (ended)
Data management concept NanoFASE The main objective of this case study was to establish a management workflow for the data-related processes at a large multidisciplinary project level, which includes the data preparation needed, the steps to introduce these processes and their benefit to the entire consortium, the establishment of communication and trust with the different partners and the training needed to familiarise the partners with the data management processes and their application. (ended)
Study design documentation This demonstration case is intended to define a way to document the study design in specific laboratories with respect to the decision making processes applied. Additionally, it will design approaches to use the study design as the basis for organising the data collection and management processes and the integration of quality assurance and control measures and their documentation. (ongoing)
SOP development documentation This demonstration case is intended to define a standardised ways to develop SOPs and share them. This will allow independent quality control and comparison as well as reuse of information as metadata accompanying datasets generated according to the SOP. (ongoing)
SbD, risk governance and nanofabrication This demonstration case is intended to optimse the ways to support data and tool sharing in different project clusters. (ongoing)
Grouping and read-across Grouping/read-across approaches constitute one key component of the complete risk assessment framework. This case is mapping the existing approaches and tools and designs roads towards integrated workflows. (ongoing)
Nanomaterial risk assessment This demonstration case will review and document existing risk assessment frameworks and checklists and develop concepts to provide interoperable tools supporting them. (ongoing)
Building regulatory acceptance In this case, selected nanoinformatics approaches will be prepared and submitted to regulatory evaluation. (ongoing)
Development of NInChI This demonstration case is will develop a new nanomaterial identifier based on the InChI concepts, the InChI for nano or NInChI. (ongoing)
References
- Papadiamantis et al., 2020: Papadiamantis, A. G.; Klaessig, F. C.; Exner, T. E.; Hofer, S.; Hofstaetter, N.; Himly, M.; Williams, M. A.; Doganis, P.; Hoover, M. D.; Afantitis, A.; Melagraki, G.; Nolan, T. S.; Rumble, J.; Maier, D.; Lynch, I. Metadata Stewardship in Nanosafety Research: Community-Driven Organisation of Metadata Schemas to Support FAIR Nanoscience Data. Nanomaterials 2020, 10 (10), 2033. https://doi.org/10.3390/nano10102033.